Microbiology
Microbiological testing includes the controlled analysis of water, beverage, food, pharmaceuticals and other consumer
products and their processing equipment to evaluate for the presence of micro-organisms that may cause harm to the
user or reduce the product quality or performance.
Microbiological testing is a crucial requirement across many industries worldwide where product, process and human health are
influenced by the presence of micro-organisms: living bacteria, viruses, yeasts and molds that are too small to be visible to the
naked eye.
International test procedures and standard lab practices have been established to provide strict methods for micro-organism
analysis and identification.
Micro-organisms can be harmful or beneficial to the product or process under analysis.
Some diseases of human, animals and plants are caused by unwanted bacteria, yeasts and mold. Other beneficial yeasts and
molds are responsible for numerous desirable processes in beer, wine, and food production and biotechnology.
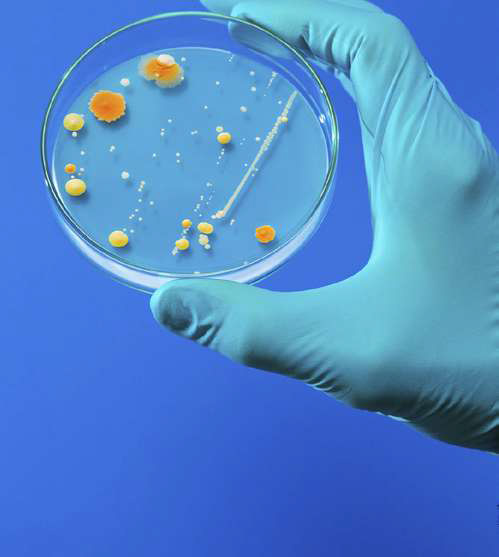
GVS products for microbiological testing include applications and testing for:
Contamination of work surfaces and equipment
Microbiological analysis of:
Potable water
Beer and wine
Waste water
Dairy products
Soft drink and concentrates
Fruit juices
Fermented products
Detection of:
Bacteria, fungi, molds
Escherichia coli (E.Coli)
Fecal streptococci and fecal coliforms
Staphylococci
Listeria
Enterococci
Pseudomonas aeruginosa
Legionella
GVS supplies a comprehensive suite of filtration and culturing products used for filtration, isolation and culturing of
samples for analysis of micro-organism presence. GVS products meet strict quality and sterility requirements and are
designed to quickly and accurately test for the presence of airborne, surface, and liquid micro-organisms.
Sterility:
For Microbiological analysis the sterility of the analysis equipment, including the membranes and culturing media, is important to
maintain a clean contaminant free zone. GVS ensures the sterility of its microbiological products through gamma radiation and
steam sterilization. All products are labeled with lot number and expiration date for additional control and security.
Product Quality Control:
Microbiological Analysis for good and bad micro-organisms present in processing, production and finished products
Contaminants and organisms are quantified and qualified at selected control points throughout the production steps and in
the finished product. The main parameter of concern depends on the industry and the finished product under review. Leading
parameters are yeast and mold, total bacteria count and E. coli as well as coliforms, fecal streptococci and Pseudomonas aeruginosa.
The Workflow for Microbiological Analysis breaks down into 5 simple steps:
1) Obtain sample for analysis
2) Filter the sample
3) Add Nutrient media
4) Incubate and culture
5) Analyze and enumerate the results
Preparation for Microbiological Analysis includes selection of:
1) membrane type and pore size
2) Nutrient media
3)Filtering Equipment
This GVS Catalog provides guidance on the selection of membranes, nutrient media and equipment and details the microbiological products available from GVS.